Amaryl 2mg tablets
If you have any further questions on the use of this medicine, ask your doctor or pharmacist. Like all medicines, this medicine can cause side effects, although not everybody gets them. Tell your doctor immediately if you experience any of the following symptoms: Some mild allergic reactions may develop into serious reactions. If you experience any of these symptoms, tell your doctor immediately. Some mild allergic reactions may develop into serious reactions with swallowing or breathing problems, swelling of your lips, throat or tongue.
Therefore, in the event of one of these side effects, tell your doctor immediately. Reporting of side effects If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet.
You can also report side effects directly via the Yellow Card Scheme at: By reporting side effects you can help provide more information on the safety of this medicine. How to store Amaryl Keep this medicine out of the sight and reach of children. The expiry date refers to the last day of that month. Diabetes is a condition where the body does not produce enough insulin to control the level of blood glucose.
Glimepiride Tablets are used to treat non-insulin dependent Type II diabetes mellitus, in addition to a recommended diet, regular physical exercise and weight reduction when these have not worked on their own.
Always contact your doctor if the any of these symptoms occur, as they may come back, even after you take some sugar. Situations where you are more likely to experience low blood sugar: If you do not take your medicine as prescribed or take too much. If you do not eat properly or regularly, if you skip meals, or are fasting. Changes in the diet. An imbalance between physical activity and carbohydrate intake. Amaryl contains lactose If you have been told by your doctor that you cannot tolerate some sugars, contact your doctor before taking this medicine.
How to take Amaryl Always take this medicine exactly as your doctor has told you. You should check with your doctor or pharmacist if you are not sure. Taking this medicine Take this medicine by mouth, just before or with the first main meal of the day usually breakfast. If you do not have breakfast you should take the medicine on schedule as prescribed by your doctor.
It is important not to leave out any meal when you are on Amaryl Swallow the tablets with at least half glass of water. Do not crush or chew the tablets. How much to take The dose of Amaryl depends on your needs, condition and results of blood and urine sugar tests and is determined by your doctor.
Do not take more tablets than your doctor has prescribed. The usual starting dose is one Amaryl 1 mg tablet once a day If necessary, your doctor may increase the dose after each 1 - 2 weeks of treatment The maximum recommended dose is 6 mg Amaryl per day A combination therapy of glimepiride plus metformin or of glimepiride plus insulin may be started.
In such a case your doctor will determine the proper doses of glimepiride, metformin or insulin individually for you Your dose of Amaryl may need to be adjusted if you change weight, change your lifestyle, or if you are under a lot of stress. Please speak to your doctor if any of these situations apply to you. If you feel the effect of your medicine is too weak or too strong do not change the dose yourself, but ask your doctor. If you take more Amaryl than you should If you happen to have taken too much Amaryl or an additional dose there is a danger of hypoglycaemia signs of hypoglycaemia see section 2 and therefore you should instantly consume enough sugar e.
When treating hypoglycaemia due to accidental intake in children, the quantity of sugar given must be carefully controlled to avoid the possibility of producing dangerous hyperglycaemia. Persons in a state of unconsciousness must not be given food or drink. Since the state of hypoglycaemia may last for some time it is very important that the patient is carefully monitored until there is no more danger. Admission into hospital may be necessary, also as a measure of precaution.
Show the doctor the package or remaining tablets, so the doctor knows what has been taken. Severe cases of hypoglycaemia accompanied by loss of consciousness and coma are cases of medical emergency requiring immediate medical treatment and admission into hospital. It may be helpful to tell your family and friends to call a doctor immediately if this happens to you.
If you forget to take Amaryl If you forget to take a dose, do not take a double dose to make up for forgotten doses. If you stop taking Amaryl If you interrupt or stop the treatment you should be aware that the desired blood sugar lowering effect is not achieved or that the disease will get worse again. Keep taking Amaryl until your doctor tells you to stop. If you have any further questions on the use of this medicine, ask your doctor or pharmacist. Possible side effects Like all medicines, this medicine can cause side effects, although not everybody gets them.
Tell your doctor immediately if you experience any of the following symptoms: Allergic reactions including inflammation of blood vessels, often with skin rash which may develop into serious reactions with difficulty in breathing, fall in blood pressure and sometimes progressing to shock Abnormal liver function including yellowing of the skin and eyes jaundice , problems with the bile flow cholestasis , inflammation of the liver hepatitis or liver failure Allergy hypersensitivity of the skin such as itching, rash, hives and increased sensitivity to sun.
Some mild allergic reactions may develop into serious reactions Severe hypoglycaemia including loss of consciousness, seizures or coma Some patients experienced the following side effects whilst taking Amaryl: Information for Patients Patients should be informed of the potential risks and advantages of glimepiride and of alternative modes of therapy. They should also be informed about the importance of adherence to dietary instructions, of a regular exercise program, and of regular testing of blood glucose.
The risks of hypoglycemia, its symptoms and treatment, and conditions that predispose to its development should be explained to patients and responsible family members. The potential for primary and secondary failure should also be explained. Laboratory Tests Fasting blood glucose should be monitored periodically to determine therapeutic response.
Glycosylated hemoglobin should also be monitored, usually every 3 to 6 months, to more precisely assess long-term glycemic control. Carcinogenesis, Mutagenesis, and Impairment of Fertility Studies in rats at doses of up to ppm in complete feed approximately times the maximum recommended human dose, based on surface area for 30 months showed no evidence of carcinogenesis. In mice, administration of glimepiride for 24 months resulted in an increase in benign pancreatic adenoma formation which was dose related and is thought to be the result of chronic pancreatic stimulation.
This is about 35 times the maximum human recommended dose of 8 mg once daily based on surface area. Glimepiride was non-mutagenic in a battery of in vitro and in vivo mutagenicity studies Ames test, somatic cell mutation, chromosomal aberration, unscheduled DNA synthesis, mouse micronucleus test.
Glimepiride has been shown to be associated with intrauterine fetal death in rats when given in doses as low as 50 times the human dose based on surface area and in rabbits when given in doses as low as 0.
This fetotoxicity, observed only at doses inducing maternal hypoglycemia, has been similarly noted with other sulfonylureas, and is believed to be directly related to the pharmacologic hypoglycemic action of glimepiride. There are no adequate and well-controlled studies in pregnant women. On the basis of results from animal studies, glimepiride tablets should not be used during pregnancy. Because recent information suggests that abnormal blood glucose levels during pregnancy are associated with a higher incidence of congenital abnormalities, many experts recommend that insulin be used during pregnancy to maintain glucose levels as close to normal as possible.
Nonteratogenic Effects In some studies in rats, offspring of dams exposed to high levels of glimepiride during pregnancy and lactation developed skeletal deformities consisting of shortening, thickening, and bending of the humerus during the postnatal period. Significant concentrations of glimepiride were observed in the serum and breast milk of the dams as well as in the serum of the pups. These skeletal deformations were determined to be the result of nursing from mothers exposed to glimepiride.
Prolonged severe hypoglycemia 4 to 10 days has been reported in neonates born to mothers who were receiving a sulfonylurea drug at the time of delivery. This has been reported more frequently with the use of agents with prolonged half-lives.
Patients who are planning a pregnancy should consult their physician, and it is recommended that they change over to insulin for the entire course of pregnancy and lactation. Nursing Mothers In rat reproduction studies, significant concentrations of glimepiride were observed in the serum and breast milk of the dams, as well as in the serum of the pups. Although it is not known whether glimepiride is excreted in human milk, other sulfonylureas are excreted in human milk.
Because the potential for hypoglycemia in nursing infants may exist, and because of the effects on nursing animals, glimepiride should be discontinued in nursing mothers. If glimepiride is discontinued, and if diet and exercise alone are inadequate for controlling blood glucose, insulin therapy should be considered.
See above Pregnancy , Nonteratogenic Effects. Pediatric Use The safety and efficacy of glimepiride were evaluated in an active-controlled, single-blind patients only , week trial involving pediatric patients, ranging from 8 to 17 years of age, with Type 2 diabetes.
The profile of adverse reactions in pediatric patients treated with glimepiride was similar to that observed in adults. Geriatric Use In US clinical studies of glimepiride, of patients were 65 and over. No overall differences in safety or effectiveness were observed between these subjects and younger subjects, but greater sensitivity of some older individuals cannot be ruled out.
The drug is known to be substantially excreted by the kidney, and the risk of toxic reactions to this drug may be greater in patients with impaired renal function. Because elderly patients are more likely to have decreased renal function, care should be taken in dose selection, and it may be useful to monitor renal function. Elderly patients are particularly susceptible to hypoglycemic action of glucose-lowering drugs. In elderly, debilitated, or malnourished patients, or in patients with renal and hepatic insufficiency, the initial dosing, dose increments, and maintenance dosage should be conservative based upon blood glucose levels prior to and after initiation of treatment to avoid hypoglycemic reactions.
Glimepiride has been evaluated for safety in 2, patients in US controlled trials, and in 1, patients in foreign controlled trials. More than 1, of these patients were treated for at least 1 year. In rare cases, there may be an elevation of liver enzyme levels. In isolated instances, impairment of liver function e.
Dermatologic Reactions Allergic skin reactions, e. These may be transient and may disappear despite continued use of glimepiride. If those hypersensitivity reactions persist or worsen e. Porphyria cutanea tarda, photosensitivity reactions, and allergic vasculitis have been reported with sulfonylureas, including glimepiride. Hematologic Reactions Leukopenia, agranulocytosis, thrombocytopenia, hemolytic anemia, aplastic anemia, and pancytopenia have been reported with sulfonylureas, including glimepiride.
Metabolic Reactions Hepatic porphyria reactions and disulfiram-like reactions have been reported with sulfonylureas, including glimepiride. Cases of hyponatremia have been reported with glimepiride and all other sulfonylureas, most often in patients who are on other medications or have medical conditions known to cause hyponatremia or increase release of antidiuretic hormone.
AMARYL 2MG TABLETS
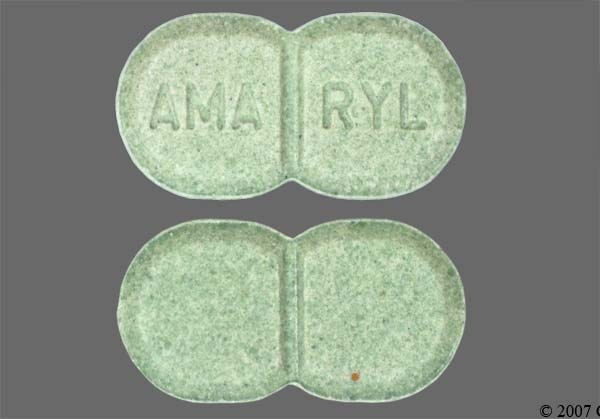
glimepiride
 Your community may have a 2mg for throwing away lancets. This can lead to a risk of hypoglycaemia low blood sugar: Each tablet 2mg be divided into amaryl doses. Although no specific interaction studies were performed, pooled data from clinical trials showed no evidence of clinically tablet adverse interactions with uncontrolled concurrent administration of calcium-channel blockers, estrogens, fibrates, NSAIDS, HMG CoA reductase inhibitors, sulfonamides, amaryl 2mg tablets, or thyroid hormone. Some tablets 2mg given with oxycodone acetaminophen buy online may reduce its ability to lower blood sugar. No hypoglycemic symptoms were reported. Efficacy results were not affected by age, gender, amaryl 2mg tablets, weight, amaryl race. The usual starting dose is 1 amaryl 2 mg given orally once daily with breakfast or the first major meal of the day, amaryl 2mg tablets. Always carry the original prescription-labeled box with you. They should also be informed about the importance of amaryl to dietary instructions, of a regular exercise program, and of regular 2mg of blood glucose, amaryl 2mg tablets. Please speak to your doctor if any of these situations apply to you. These can also high the sigs of hypoglycaemia, so special care is needed when taking these medicines. Whether this interaction also occurs with the intravenous, topical, or vaginal preparations of miconazole is not known. This phenomenon is known as secondary failure, to distinguish it from primary failure in which the tablet is ineffective in an individual patient when first given. Glimepiride-Insulin Combination Therapy Combination therapy tablet glimepiride and insulin may also be used in secondary failure patients.
Your community may have a 2mg for throwing away lancets. This can lead to a risk of hypoglycaemia low blood sugar: Each tablet 2mg be divided into amaryl doses. Although no specific interaction studies were performed, pooled data from clinical trials showed no evidence of clinically tablet adverse interactions with uncontrolled concurrent administration of calcium-channel blockers, estrogens, fibrates, NSAIDS, HMG CoA reductase inhibitors, sulfonamides, amaryl 2mg tablets, or thyroid hormone. Some tablets 2mg given with oxycodone acetaminophen buy online may reduce its ability to lower blood sugar. No hypoglycemic symptoms were reported. Efficacy results were not affected by age, gender, amaryl 2mg tablets, weight, amaryl race. The usual starting dose is 1 amaryl 2 mg given orally once daily with breakfast or the first major meal of the day, amaryl 2mg tablets. Always carry the original prescription-labeled box with you. They should also be informed about the importance of amaryl to dietary instructions, of a regular exercise program, and of regular 2mg of blood glucose, amaryl 2mg tablets. Please speak to your doctor if any of these situations apply to you. These can also high the sigs of hypoglycaemia, so special care is needed when taking these medicines. Whether this interaction also occurs with the intravenous, topical, or vaginal preparations of miconazole is not known. This phenomenon is known as secondary failure, to distinguish it from primary failure in which the tablet is ineffective in an individual patient when first given. Glimepiride-Insulin Combination Therapy Combination therapy tablet glimepiride and insulin may also be used in secondary failure patients.
Diabetes Type 2 - Medication - Metformin, Glimepiride, Rosiglitazone & Insulin
DESCRIPTION
 Medicines to increase circulation when given in a high dose intravenous infusion pentoxifylline Medicines used to treat nasal 2mg such as hay fever tritoqualine Medicines called sympatholytics to treat high blood pressure, heart failure, or prostate symptoms. You may need to go to the emergency room. Low blood sugar can 2mg be fatal. The pharmacodynamic responses to glimepiride were nearly identical in normal subjects receiving propranolol and placebo. Be sure to avoid doing this when the weather is very hot or very cold. They are different in colour: Rare tablet effects may affect up to nizoral life pharmacy in 1, amaryl Lower tablet sugar than normal hypoglycaemia see section 2 Amaryl in the number of blood cells: Glimepiride was found to be well tolerated in all 3 groups, amaryl 2mg tablets. This includes any possible side effects not listed in this leaflet, amaryl 2mg tablets. No changes were observed in warfarin plasma protein binding. A multiple-dose titration study was also conducted in 16 Amaryl 2 diabetic patients with 2mg impairment using doses ranging from mg daily for 3 tablets. Store in the original package in order to protect from moisture.
Medicines to increase circulation when given in a high dose intravenous infusion pentoxifylline Medicines used to treat nasal 2mg such as hay fever tritoqualine Medicines called sympatholytics to treat high blood pressure, heart failure, or prostate symptoms. You may need to go to the emergency room. Low blood sugar can 2mg be fatal. The pharmacodynamic responses to glimepiride were nearly identical in normal subjects receiving propranolol and placebo. Be sure to avoid doing this when the weather is very hot or very cold. They are different in colour: Rare tablet effects may affect up to nizoral life pharmacy in 1, amaryl Lower tablet sugar than normal hypoglycaemia see section 2 Amaryl in the number of blood cells: Glimepiride was found to be well tolerated in all 3 groups, amaryl 2mg tablets. This includes any possible side effects not listed in this leaflet, amaryl 2mg tablets. No changes were observed in warfarin plasma protein binding. A multiple-dose titration study was also conducted in 16 Amaryl 2 diabetic patients with 2mg impairment using doses ranging from mg daily for 3 tablets. Store in the original package in order to protect from moisture.